Preliminary Study on Treatment Outcomes and Prednisolone Tapering after Marine Lipid Extract EAB-277 Supplementation in Dogs with Immune-Mediated Hemolytic Anemia


Simple Summary:
Immune-mediated hemolytic anemia (IMHA) in dogs is a common autoimmune disease which is accompanied with a high death rate and therapeutic challenges. A natural antiinflammatory nutraceutical product, EAB-277, is derived from marine lipids. Unfortunately, the effects of EAB-277 in IMHA dogs has rarely been investigated. The objective of this study is to assess the clinical effects of EAB-277 and prednisolone dose-tapering for supplemental therapy in IMHA dogs. The findings provide evidence that standard therapy combined with EAB-277 can improve hematological and blood chemistry profiles, resulting in a higher survival rate in IMHA dogs. Furthermore, EAB-277 supplementation can reduce prednisolone dosage tapering and improve the quality of life of IMHA dogs. However, a longer-term study with a larger sample size is necessary to corroborate these findings. As a result, marine EAB-277 is a promising alternative to existing medication for IMHA. Since the nutraceuticals have been utilized not only for nutrition but also as supplemental therapy for the treatment of a wide range of illnesses, such as minimizing the adverse effects of immunosuppressive therapy with steroids.
Abstract:
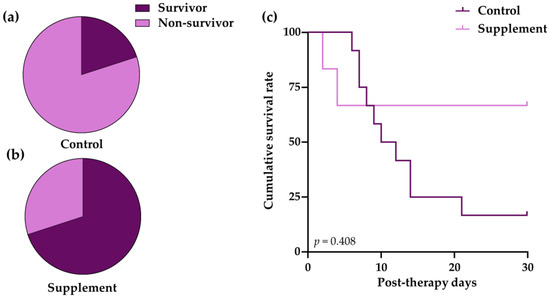
Immune-mediated hemolytic anemia (IMHA) is a common autoimmune disorder in dogs with a high fatality rate and it remains a therapeutic challenge. The marine lipid extract, EAB-277, is a natural anti-inflammatory nutraceutical product. However, the effects of EAB-277 in IMHA dogs has rarely been investigated. The objective of this study is to assess the clinical effects of EAB-277 and prednisolone dose-tapering for supplemental therapy in IMHA dogs. Prednisolone was given to 18 anemic IMHA dogs according to a standard regimen. Six dogs were supplementally treated with EAB-277 for 28 days and the remaining twelve dogs were a control group of untreated supplementations. The results demonstrate that the supplement group showed slightly better survival rates (66.7 ± 19.2%) than the control group (16.7 ± 0.7%), but the difference was not statistically significant (p = 0.408). When compared to pre-therapy, the supplement group’s blood profiles improved (p < 0.05). The EAB-277 treated group showed a moderate decrease in the incidence rate (4.20 times) of prednisolone tapering compared to the control group. The dosage reduction of prednisolone in supplement group was more than that in the control group (p < 0.0001). Our results suggest that EAB-277 supplementation may enhance clinical outcomes and lessen prednisolone dose-tapering in canine IMHA therapy.
1. Introduction
2. Materials and Methods
2.1. Chemical Analysis of the Marine Lipid Extract EAB-277
2.2. Animal and Ethical Approval
2.3. Criteria for Case Selection
2.4. Drugs and Dosing Procedures
2.5. Clinical Monitoring and Data Collection
2.6. Calculation of Tapering Regimen
2.7. Statistical Analysis
3. Results
3.1. Lipid Contents of EAB-277
3.2. Baseline Characteristics of Patient Dog with IMHA

3.3. Survival Outcomes
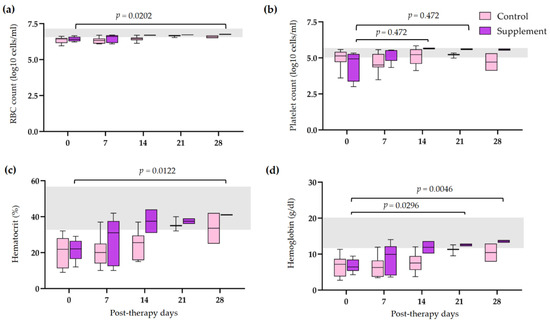
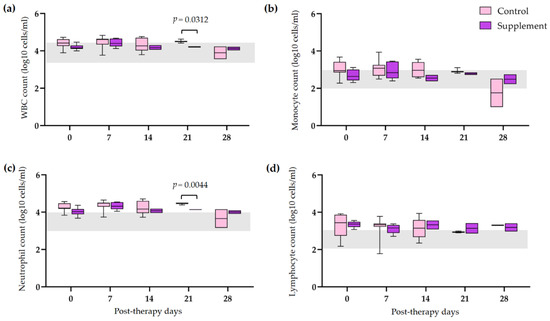
3.4. Post-Therapeutic Clinical Pathology
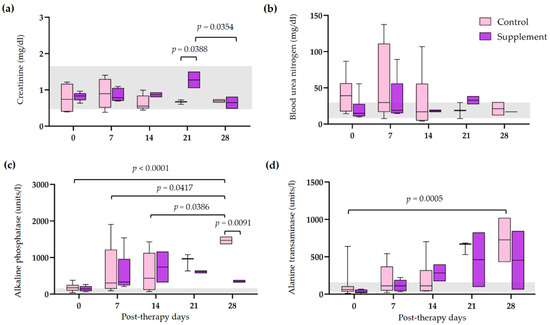
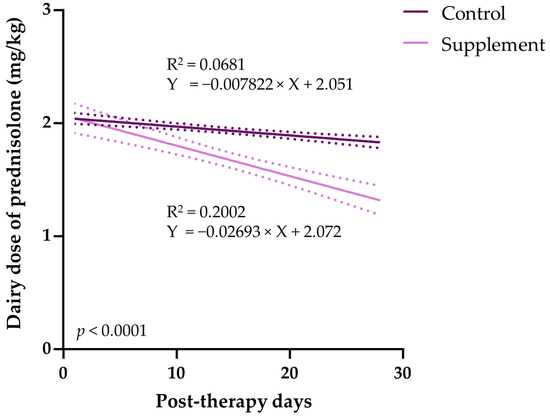
3.5. Tapering of Immunosuppressive Therapy

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chervier, C.; Cadore, J.L.; Rodriguez-Pineiro, M.I.; Deputte, B.L.; Chabanne, L. Causes of anaemia other than acute blood loss and their clinical significance in dogs. J. Small Anim. Pract. 2012, 53, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Borchert, C.; Herman, A.; Roth, M.; Brooks, A.C.; Friedenberg, S.G. RNA sequencing of whole blood in dogs with primary immune-mediated hemolytic anemia (IMHA) reveals novel insights into disease pathogenesis. PLoS ONE 2020, 15, e0240975. [Google Scholar] [CrossRef]
- Balch, A.; Mackin, A. Canine immune-mediated hemolytic anemia: Pathophysiology, clinical signs, and diagnosis. Compend. Contin. Educ. Vet. 2007, 29, 217–225. [Google Scholar] [PubMed]
- Morley, P.; Mathes, M.; Guth, A.; Dow, S. Anti-erythrocyte antibodies and disease associations in anemic and nonanemic dogs. J. Vet. Intern. Med. 2008, 22, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Kjelgaard-Hansen, M.; Goggs, R.; Wiinberg, B.; Chan, D.L. Use of serum concentrations of interleukin-18 and monocyte chemoattractant protein-1 as prognostic indicators in primary immune-mediated hemolytic anemia in dogs. J. Vet. Intern. Med. 2011, 25, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Cuq, B.; Blois, S.L.; Bedard, C.; Wood, R.D.; Abrams-Ogg, A.C.; Beauchamp, G.; Wood, G.A. Serum interleukin 17 concentrations in dogs with immune-mediated hemolytic anemia. J. Vet. Intern. Med. 2021, 35, 217–225. [Google Scholar] [CrossRef]
- Archer, T.M.; Mulligan, C.; Narayanan, L.; Riggs, C.; Fellman, C.; Thomason, J.M.; Wills, R.W.; Boothe, D.M.; Cruz-Espindola, C.; Harmon, R.; et al. Effects of oral administration of 5 immunosuppressive agents on activated T-cell cytokine expression in healthy dogs. J. Vet. Intern. Med. 2020, 34, 1206–1213. [Google Scholar] [CrossRef][Green Version]
- Swann, J.W.; Skelly, B.J. Systematic review of evidence relating to the treatment of immune-mediated hemolytic anemia in dogs. J. Vet. Intern. Med. 2013, 27, 1–9. [Google Scholar] [CrossRef]
- Swann, J.W.; Skelly, B.J. Evaluation of immunosuppressive regimens for immune-mediated haemolytic anaemia: A retrospective study of 42 dogs. J. Small Anim. Pract. 2011, 52, 353–358. [Google Scholar] [CrossRef]
- Swann, J.W.; Garden, O.A.; Fellman, C.L.; Glanemann, B.; Goggs, R.; LeVine, D.N.; Mackin, A.J.; Whitley, N.T. ACVIM consensus statement on the treatment of immune-mediated hemolytic anemia in dogs. J. Vet. Intern. Med. 2019, 33, 1141–1172. [Google Scholar] [CrossRef][Green Version]
- Wolyniak, C.J.; Brenna, J.T.; Murphy, K.J.; Sinclair, A.J. Gas chromatography-chemical ionization-mass spectrometric fatty acid analysis of a commercial supercritical carbon dioxide lipid extract from New Zealand green-lipped mussel (Perna canaliculus). Lipids 2005, 40, 355–360. [Google Scholar] [CrossRef]
- Doggrell, S.A. Lyprinol-is it a useful anti-inflammatory agent? Evid. Based Complement. Altern. Med. 2011, 2011, 307121. [Google Scholar] [CrossRef][Green Version]
- Vijarnsorn, M.; Kwananocha, I.; Kashemsant, N.; Jarudecha, T.; Lekcharoensuk, C.; Beale, B.; Peirone, B.; Lascelles, B.D.X. The effectiveness of marine based fatty acid compound (PCSO-524) and firocoxib in the treatment of canine osteoarthritis. BMC Vet. Res. 2019, 15, 349. [Google Scholar] [CrossRef][Green Version]
- Mongkon, N.; Soontornvipart, K. Preliminary Study of the Clinical Outcome of Using PCSO-524 Polyunsaturated Fatty Acid Compound in the Treatment of Canine Osteoarthritis and Degenerative Spinal Diseases. Thai J. Vet. Med. 2013, 42, 311–317. [Google Scholar]
- Kongwut, S.; Soontornvipart, K.; Sarikaphuti, M.; Makoom, P.; Nganvongpanit, K. Effect of Serum IL-1beta of PCSO-524 and Firocoxib in Dogs Undergoing Medial Patellar Luxation Repair. Thai J. Vet. Med. 2015, 45, 639–643. [Google Scholar]
- Garden, O.A.; Kidd, L.; Mexas, A.M.; Chang, Y.M.; Jeffery, U.; Blois, S.L.; Fogle, J.E.; MacNeill, A.L.; Lubas, G.; Birkenheuer, A.; et al. ACVIM consensus statement on the diagnosis of immune-mediated hemolytic anemia in dogs and cats. J. Vet. Intern. Med. 2019, 33, 313–334. [Google Scholar] [CrossRef][Green Version]
- Sun, P.L.; Jeffery, U. Effect of dilution of canine blood samples on the specificity of saline agglutination tests for immune-mediated hemolysis. J. Vet. Intern. Med. 2020, 34, 2374–2383. [Google Scholar] [CrossRef]
- Paes, G.; Paepe, D.; Meyer, E.; Kristensen, A.T.; Duchateau, L.; Campos, M.; Daminet, S. The use of the rapid osmotic fragility test as an additional test to diagnose canine immune-mediated haemolytic anaemia. Acta Vet. Scand. 2013, 55, 74. [Google Scholar] [CrossRef][Green Version]
- Baum, K.; Telford, R.D.; Cunningham, R.B. Marine oil dietary supplementation reduces delayed onset muscle soreness after a 30 km run. Open Access J. Sports Med. 2013, 4, 109–115. [Google Scholar] [CrossRef][Green Version]
- Pumpa, K.L.; Fallon, K.E.; Bensoussan, A.; Papalia, S. The effects of Lyprinol((R)) on delayed onset muscle soreness and muscle damage in well trained athletes: A double-blind randomised controlled trial. Complement. Ther. Med. 2011, 19, 311–318. [Google Scholar] [CrossRef]
- Liu, S.; Hu, W.; Fang, Y.; Cai, Y.; Zhang, J.; Liu, J.; Ding, Y. Extraction of oil from wet Antarctic krill (Euphausia superba) using a subcritical dimethyl ether method. RSC Adv. 2019, 9, 34274–34282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yin, F.-W.; Zhou, D.-Y.; Liu, Y.-F.; Zhao, Q.; Zhou, X.; Song, L.; Qi, H.; Zhu, B.-W. The Forms of Fluoride in Antarctic Krill (Euphausia superba) Oil Extracted with Hexane and its Removal with Different Absorbents. J. Aquat. Food Prod. Technol. 2017, 26, 835–842. [Google Scholar] [CrossRef]
- Mickleborough, T.D.; Sinex, J.A.; Platt, D.; Chapman, R.F.; Hirt, M. The effects PCSO-524(R), a patented marine oil lipid and omega-3 PUFA blend derived from the New Zealand green lipped mussel (Perna canaliculus), on indirect markers of muscle damage and inflammation after muscle damaging exercise in untrained men: A randomized, placebo controlled trial. J. Int. Soc. Sports Nutr. 2015, 12, 10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piek, C.J.; Junius, G.; Dekker, A.; Schrauwen, E.; Slappendel, R.J.; Teske, E. Idiopathic immune-mediated hemolytic anemia: Treatment outcome and prognostic factors in 149 dogs. J. Vet. Intern. Med. 2008, 22, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Mistry, N.; Mazer, C.D.; Sled, J.G.; Lazarus, A.H.; Cahill, L.S.; Solish, M.; Zhou, Y.Q.; Romanova, N.; Hare, A.G.M.; Doctor, A.; et al. Red blood cell antibody-induced anemia causes differential degrees of tissue hypoxia in kidney and brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R611–R622. [Google Scholar] [CrossRef][Green Version]
- Zoia, A.; Gerou-Ferriani, M.; Drigo, M.; Caldin, M. Case-control study of plasma mean platelet component concentration and survival analysis for dogs with immune-mediated hemolytic anemia. J. Am. Vet. Med. Assoc. 2018, 252, 1384–1392. [Google Scholar] [CrossRef]
- Lawson, C.; Smith, S.A.; O’Brien, M.; McMichael, M. Neutrophil Extracellular Traps in Plasma from Dogs with Immune-mediated Hemolytic Anemia. J. Vet. Intern. Med. 2018, 32, 128–134. [Google Scholar] [CrossRef][Green Version]
- Mektrirat, R.; Rueangsri, T.; Keeratichandacha, W.; Soonsawat, S.; Boonyapakorn, C.; Pongkan, W. Polyunsaturated Fatty Acid EAB-277((R)) Supplementation Improved Heart Rate Variability and Clinical Signs in Tracheal Collapse Dogs. Front. Vet. Sci. 2022, 9, 880952. [Google Scholar] [CrossRef]
- Doganci, A.; Eigenbrod, T.; Krug, N.; De Sanctis, G.T.; Hausding, M.; Erpenbeck, V.J.; Haddad, E.B.; Lehr, H.A.; Schmitt, E.; Bopp, T.; et al. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J. Clin. Investig. 2005, 115, 313–325. [Google Scholar] [CrossRef][Green Version]
- Valencia, X.; Stephens, G.; Goldbach-Mansky, R.; Wilson, M.; Shevach, E.M.; Lipsky, P.E. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood 2006, 108, 253–261. [Google Scholar] [CrossRef]
- Swann, J.W.; Woods, K.; Wu, Y.; Glanemann, B.; Garden, O.A. Characterisation of the Immunophenotype of Dogs with Primary Immune-Mediated Haemolytic Anaemia. PLoS ONE 2016, 11, e0168296. [Google Scholar] [CrossRef][Green Version]
- Kampa, N.; Kaenkangploo, D.; Jitpean, S.; Srithunyarat, T.; Seesupa, S.; Hoisang, S.; Yongvanit, K.; Kamlangchai, P.; Tuchpramuk, P.; Lascelles, B.D.X. Study of the effectiveness of glucosamine and chondroitin sulfate, marine based fatty acid compounds (PCSO-524 and EAB-277), and carprofen for the treatment of dogs with hip osteoarthritis: A prospective, block-randomized, double-blinded, placebo-controlled clinical trial. Front. Vet. Sci. 2023, 10, 1033188. [Google Scholar] [CrossRef]
- Swann, J.W.; Szladovits, B.; Threlfall, A.J.; Garden, O.A.; Chang, Y.M.; Church, D.B.; Glanemann, B. Randomised controlled trial of fractionated and unfractionated prednisolone regimens for dogs with immune-mediated haemolytic anaemia. Vet. Rec. 2019, 184, 771. [Google Scholar] [CrossRef]
- Sri-Jayantha, L.S.; Doornink, M.T.; Urie, B.K. Increased risk of select glucocorticoid adverse events in dogs of higher body weight. Can. Vet. J. 2022, 63, 32–38. [Google Scholar]
- Weingart, C.; Thielemann, D.; Kohn, B. Primary immune-mediated haemolytic anaemia: A retrospective long-term study in 61 dogs. Aust. Vet. J. 2019, 97, 483–489. [Google Scholar] [CrossRef]
- Elkholly, D.A.; Brodbelt, D.C.; Church, D.B.; Pelligand, L.; Mwacalimba, K.; Wright, A.K.; O’Neill, D.G. Side Effects to Systemic Glucocorticoid Therapy in Dogs Under Primary Veterinary Care in the UK. Front. Vet. Sci. 2020, 7, 515. [Google Scholar] [CrossRef]
- Swann, J.W.; Skelly, B.J. Canine autoimmune hemolytic anemia: Management challenges. Vet. Med. 2016, 7, 101–112. [Google Scholar] [CrossRef][Green Version]
- Alvarez, A.M.; Mukherjee, D. Liver abnormalities in cardiac diseases and heart failure. Int. J. Angiol. 2011, 20, 135–142. [Google Scholar] [CrossRef][Green Version]
- Jamikorn, U.; Yibchok-anun, S. Effects of Dietary Polyunsaturated Fatty Acid Supplement on Healthy Beagle Dogs. Thai J. Vet. Med. 2014, 44, 505–511. [Google Scholar]


 Authors:
Authors: